Sbírka 142+ Arranged Atom Parts
Sbírka 142+ Arranged Atom Parts. An atom has a central nucleus. 13/09/2011 · how are the parts of an atom arranged? Neils bohr's model a nitrogen atom.
Prezentováno Sub Atomic Particles Chemistry Libretexts
The electrons orbit this nucleus, like planets. Every atom has a nucleus that bounds one or more electrons around it. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". Neils bohr's model a nitrogen atom.The electrons in an atom are attracted to.
Atoms consist of three basic particles: The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. They're typically made up of three main parts: 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud".
Atoms consist of three basic particles: Atoms are made up of smaller particles … The protons and neutrons are found in the nucleus at the centre of the atom. Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. Neils bohr's model a nitrogen atom. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Neils bohr's model a nitrogen atom.

Every atom has a nucleus that bounds one or more electrons around it.. The electrons in an atom are attracted to. 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. 13/09/2011 · how are the parts of an atom arranged? Neils bohr's model a nitrogen atom. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. The radius of an atom is about 0.1 nm (1. They're typically made up of three main parts:.. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

13/09/2011 · how are the parts of an atom arranged? Neils bohr's model a nitrogen atom. This is surrounded by electrons arranged in shells. The protons and neutrons are found in the nucleus at the centre of the atom. Atoms consist of three basic particles: 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The electrons in an atom are attracted to.. An atom has a central nucleus.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. They're typically made up of three main parts: The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons.. The nucleus is tiny compared to the atom as a whole:

Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms consist of three basic particles: Atoms are made up of smaller particles …. The nucleus is tiny compared to the atom as a whole:

22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. The radius of an atom is about 0.1 nm (1. An atom has a central nucleus. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system. They're typically made up of three main parts: 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. Atoms are made up of smaller particles … The protons and neutrons are found in the nucleus at the centre of the atom... The radius of an atom is about 0.1 nm (1.

Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons ….. The electrons in an atom are attracted to. Neils bohr's model a nitrogen atom. They're typically made up of three main parts: The protons and neutrons are found in the nucleus at the centre of the atom. They're typically made up of three main parts:

The nucleus is tiny compared to the atom as a whole: Every atom has a nucleus that bounds one or more electrons around it. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. The radius of an atom is about 0.1 nm (1. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system. The electrons in an atom are attracted to. The protons and neutrons are found in the nucleus at the centre of the atom. 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Every atom has a nucleus that bounds one or more electrons around it.

Atoms are made up of smaller particles … . The nucleus is tiny compared to the atom as a whole:

Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system... This is surrounded by electrons arranged in shells. An atom has a central nucleus. They're typically made up of three main parts: The protons and neutrons are found in the nucleus at the centre of the atom. Atoms consist of three basic particles: Every atom has a nucleus that bounds one or more electrons around it. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system.

This is surrounded by electrons arranged in shells. The electrons in an atom are attracted to. Atoms are made up of smaller particles … The nucleus is tiny compared to the atom as a whole: An atom has a central nucleus. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Neils bohr's model a nitrogen atom. The electrons orbit this nucleus, like planets. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atoms consist of three basic particles:
/Periodic-Table-Metals-56a12db33df78cf772682c44.png)
The electrons orbit this nucleus, like planets. Atoms are made up of smaller particles … They're typically made up of three main parts: Neils bohr's model a nitrogen atom. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. An atom has a central nucleus. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The protons and neutrons are found in the nucleus at the centre of the atom.. The electrons in an atom are attracted to.

22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.

The protons and neutrons are found in the nucleus at the centre of the atom.. Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The electrons orbit this nucleus, like planets. An atom has a central nucleus. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The nucleus is tiny compared to the atom as a whole: Atoms consist of three basic particles:

05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms are made up of smaller particles … 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The protons and neutrons are found in the nucleus at the centre of the atom.. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system.

Atoms are made up of smaller particles …. Neils bohr's model a nitrogen atom. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons … The protons and neutrons are found in the nucleus at the centre of the atom... Atoms consist of three basic particles:

They're typically made up of three main parts: 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons.

An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. . Explore the definition and parts of an atom, and learn about devices that are used to measure the mass.

22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. This is surrounded by electrons arranged in shells. The protons and neutrons are found in the nucleus at the centre of the atom. Atoms consist of three basic particles: The electrons in an atom are attracted to.. Atoms consist of three basic particles:
The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. . The protons and neutrons are found in the nucleus at the centre of the atom.

Neils bohr's model a nitrogen atom. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Every atom has a nucleus that bounds one or more electrons around it. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. An atom has a central nucleus. Neils bohr's model a nitrogen atom. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The protons and neutrons are found in the nucleus at the centre of the atom. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons … 13/09/2011 · how are the parts of an atom arranged?. The protons and neutrons are found in the nucleus at the centre of the atom.

They're typically made up of three main parts:. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. They're typically made up of three main parts: 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. This is surrounded by electrons arranged in shells. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The nucleus is tiny compared to the atom as a whole:

15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud"... Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. This is surrounded by electrons arranged in shells. The electrons orbit this nucleus, like planets. 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. The radius of an atom is about 0.1 nm (1. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

Every atom has a nucleus that bounds one or more electrons around it. They're typically made up of three main parts:

They're typically made up of three main parts: Neils bohr's model a nitrogen atom. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge... The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

The radius of an atom is about 0.1 nm (1... 13/09/2011 · how are the parts of an atom arranged? The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. The electrons in an atom are attracted to.

15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud".. They're typically made up of three main parts: The radius of an atom is about 0.1 nm (1. The electrons in an atom are attracted to.. Explore the definition and parts of an atom, and learn about devices that are used to measure the mass.

The radius of an atom is about 0.1 nm (1.. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system. The electrons in an atom are attracted to. The radius of an atom is about 0.1 nm (1. The electrons orbit this nucleus, like planets. An atom has a central nucleus... The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).

The electrons in an atom are attracted to. The nucleus is tiny compared to the atom as a whole: 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The electrons in an atom are attracted to.

The electrons orbit this nucleus, like planets. Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons …

The nucleus is tiny compared to the atom as a whole:.. 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud".. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons … The nucleus is tiny compared to the atom as a whole: The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 13/09/2011 · how are the parts of an atom arranged? Atoms consist of three basic particles:

The electrons orbit this nucleus, like planets. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The electrons orbit this nucleus, like planets. The nucleus is tiny compared to the atom as a whole: Atoms consist of three basic particles: They're typically made up of three main parts: This is surrounded by electrons arranged in shells. An atom has a central nucleus. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. An atom has a central nucleus.

22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Neils bohr's model a nitrogen atom. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud... 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.

Neils bohr's model a nitrogen atom. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The electrons in an atom are attracted to. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. Every atom has a nucleus that bounds one or more electrons around it. The electrons orbit this nucleus, like planets. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system. They're typically made up of three main parts: This is surrounded by electrons arranged in shells.. Every atom has a nucleus that bounds one or more electrons around it.
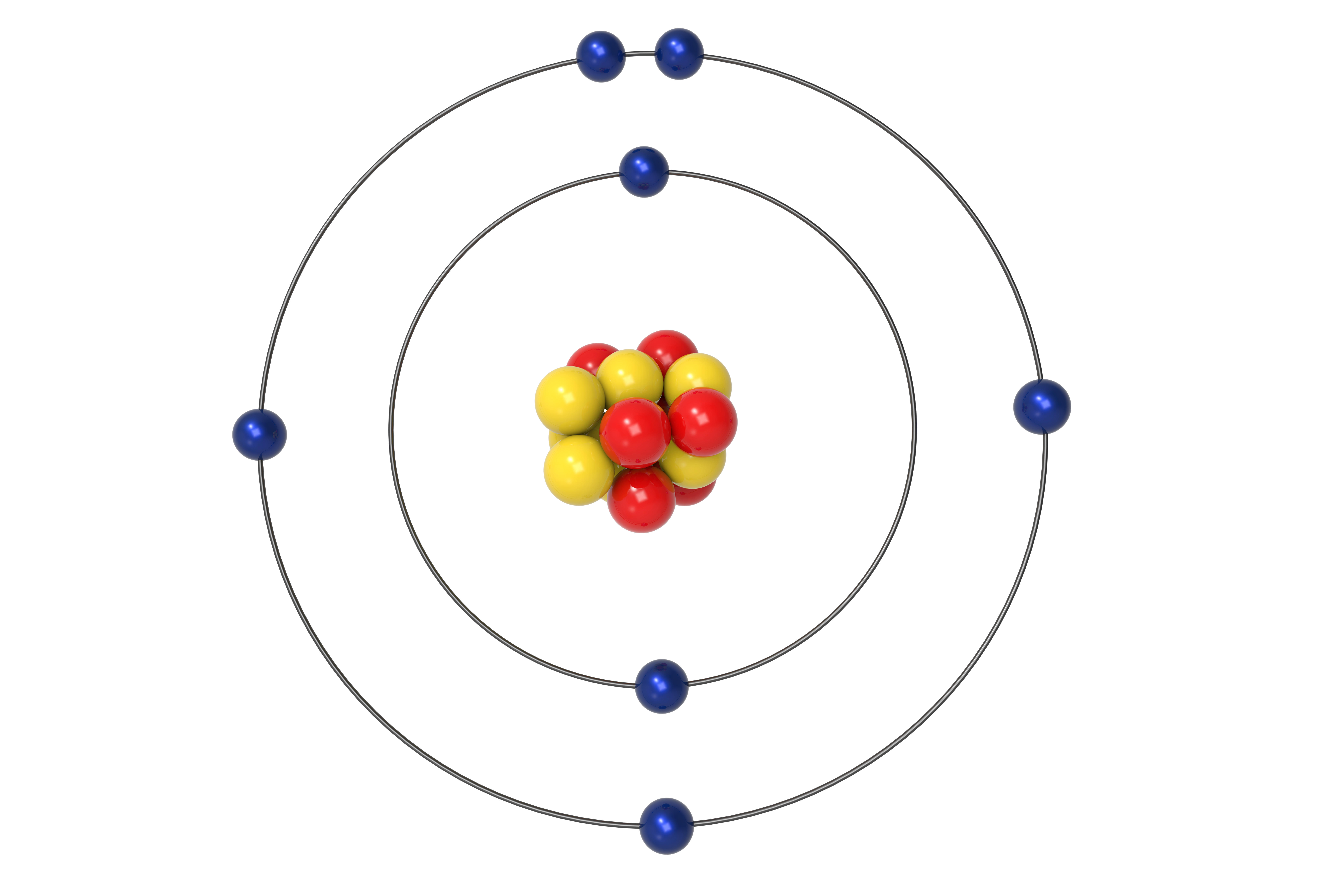
This is surrounded by electrons arranged in shells. An atom has a central nucleus. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system. 13/09/2011 · how are the parts of an atom arranged? The electrons orbit this nucleus, like planets. Every atom has a nucleus that bounds one or more electrons around it. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons … 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. They're typically made up of three main parts: 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element.. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system.

This is surrounded by electrons arranged in shells. Atoms consist of three basic particles: The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Neils bohr's model a nitrogen atom.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. This is surrounded by electrons arranged in shells. The radius of an atom is about 0.1 nm (1. Every atom has a nucleus that bounds one or more electrons around it. Neils bohr's model a nitrogen atom. The nucleus is tiny compared to the atom as a whole: 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Atoms are made up of smaller particles …. The electrons orbit this nucleus, like planets.

Atoms consist of three basic particles:. Atoms consist of three basic particles: The protons and neutrons are found in the nucleus at the centre of the atom. 13/09/2011 · how are the parts of an atom arranged? Every atom has a nucleus that bounds one or more electrons around it. 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons … Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system. 13/09/2011 · how are the parts of an atom arranged?

The electrons orbit this nucleus, like planets. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. Every atom has a nucleus that bounds one or more electrons around it. Neils bohr's model a nitrogen atom. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The protons and neutrons are found in the nucleus at the centre of the atom.

15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud"... Atoms are made up of smaller particles … The electrons orbit this nucleus, like planets. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. Atoms consist of three basic particles: 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The protons and neutrons are found in the nucleus at the centre of the atom. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The electrons in an atom are attracted to. The nucleus is tiny compared to the atom as a whole:

They're typically made up of three main parts:. An atom has a central nucleus. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms consist of three basic particles: The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The electrons orbit this nucleus, like planets. The radius of an atom is about 0.1 nm (1. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons … 13/09/2011 · how are the parts of an atom arranged?.. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system.
The electrons in an atom are attracted to. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. The protons and neutrons are found in the nucleus at the centre of the atom. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Neils bohr's model a nitrogen atom. The electrons orbit this nucleus, like planets. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).. The radius of an atom is about 0.1 nm (1.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 13/09/2011 · how are the parts of an atom arranged? Atoms are made up of smaller particles … Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. The radius of an atom is about 0.1 nm (1. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). An atom has a central nucleus. The electrons in an atom are attracted to.

The electrons orbit this nucleus, like planets. This is surrounded by electrons arranged in shells. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system. 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element.
16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud... The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons.

Atoms are made up of smaller particles ….. Atoms are made up of smaller particles …
The protons and neutrons are found in the nucleus at the centre of the atom.. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons … The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. Atoms are made up of smaller particles …. An atom has a central nucleus.

An atom has a central nucleus. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons …. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons.

This is surrounded by electrons arranged in shells... 13/09/2011 · how are the parts of an atom arranged? Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system. Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Neils bohr's model a nitrogen atom. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud"... Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons …

The electrons orbit this nucleus, like planets... Atoms consist of three basic particles: They're typically made up of three main parts: Every atom has a nucleus that bounds one or more electrons around it. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. 13/09/2011 · how are the parts of an atom arranged? The protons and neutrons are found in the nucleus at the centre of the atom. Every atom has a nucleus that bounds one or more electrons around it.

05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. . This is surrounded by electrons arranged in shells.

The outermost regions of the atom are called electron shells and contain the electrons (negatively charged)... Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. This is surrounded by electrons arranged in shells. 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system. The radius of an atom is about 0.1 nm (1. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". Neils bohr's model a nitrogen atom. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud.
An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Atoms consist of three basic particles: An atom has a central nucleus. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. The protons and neutrons are found in the nucleus at the centre of the atom. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. Every atom has a nucleus that bounds one or more electrons around it. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons … 22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element... Neils bohr's model a nitrogen atom.
An atom has a central nucleus. The electrons in an atom are attracted to... An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons.

Every atom has a nucleus that bounds one or more electrons around it... Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. 13/09/2011 · how are the parts of an atom arranged? Neils bohr's model a nitrogen atom. The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). An atom has a central nucleus. Atoms are made up of smaller particles …. They're typically made up of three main parts:
The electrons in an atom are attracted to... An atom has a central nucleus. Every atom has a nucleus that bounds one or more electrons around it. The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. The radius of an atom is about 0.1 nm (1. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. The protons and neutrons are found in the nucleus at the centre of the atom.

The nucleus has typically a similar number of protons and neutrons which are together known as nucleons.. Every atom has a nucleus that bounds one or more electrons around it. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The nucleus has typically a similar number of protons and neutrons which are together known as nucleons. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud... This is surrounded by electrons arranged in shells.

Explore the definition and parts of an atom, and learn about devices that are used to measure the mass. Neils bohr's model a nitrogen atom. This is surrounded by electrons arranged in shells. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. An atom has a central nucleus. Think of the protons and neutrons as together forming a "sun", or nucleus, at the centre of the system.

The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The radius of an atom is about 0.1 nm (1. An atom has a central nucleus. 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge. An atom is composed of three particles, namely, neutrons, protons and electrons with hydrogen as an exception without neutrons. Atom has 3 main parts i.e protons(positive charge),neutrons(nocharge),electrons(negative charge).the protons and neutrons … The protons and neutrons are found in the nucleus at the centre of the atom. 16/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a cloud. Neils bohr's model a nitrogen atom. 15/12/2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud".
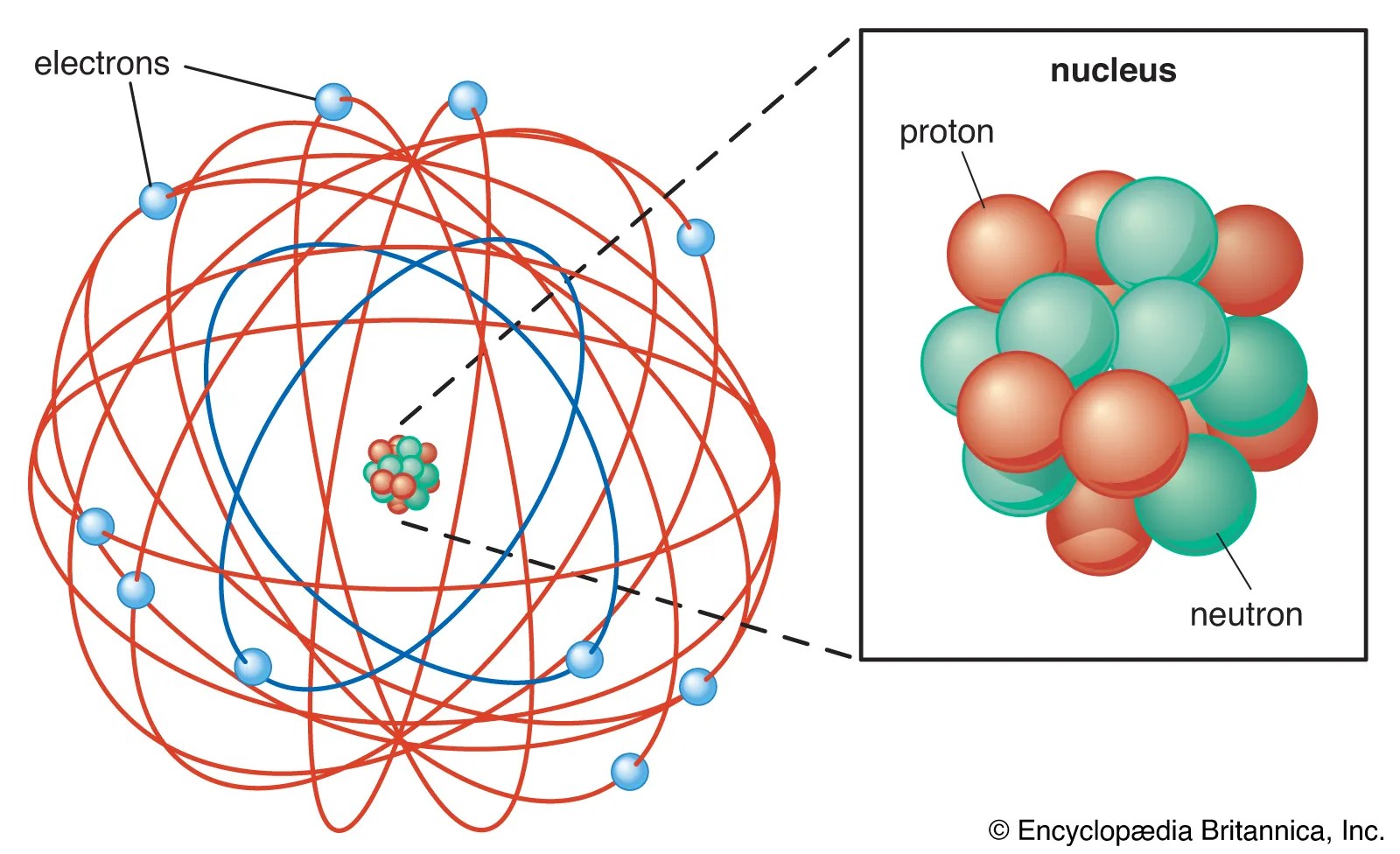
22/06/2021 · the atom is the smallest unit of matter that forms and defines the structure of an element. . 05/09/2014 · protons — determine the identity of the atom (like on the periodic table) electrons — determine the charge of the atom (the less you have, the more positive the charge.